
TruSight Cystic Fibrosis
FDA-regulated, CE-marked in vitro diagnostic (IVD) next-generation sequencing assay conveniently providing two cystic fibrosis tests in one product.
Solutions for IVD workflow and clinical research
NGS-based IVD kits enabling clinical diagnostics, assay development, and clinical research across multiple applications

Laboratory information management systems (LIMS)
Understand what a LIMS is, and how it can help genomics labs with sample tracking, workflow automation, and downstream data analysis.
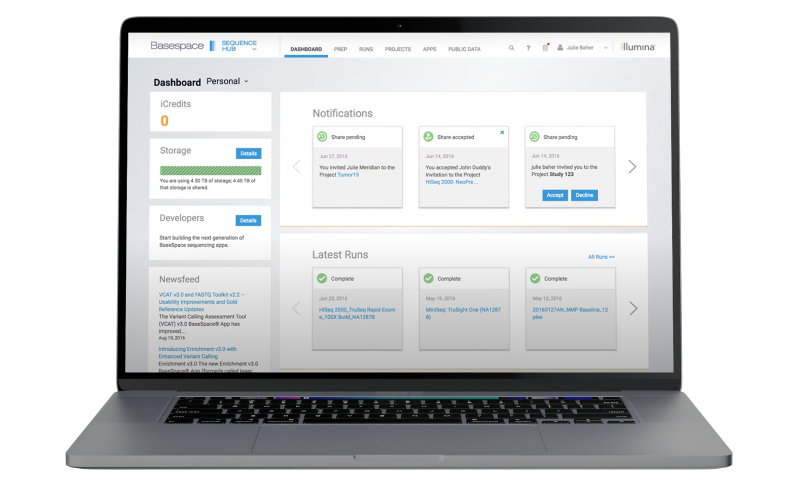
The Illumina genomics cloud-computing environment for NGS data analysis and management.
Our variant annotation and analysis software tools can help researchers extract and report biological insights from large volumes of genomic data.
An interactive omics knowledge base and data search engine that puts private data into biological context with highly curated public omics data.
This remote instrument performance monitoring service helps you minimize unscheduled downtime for your Illumina sequencing systems.
Qualify Illumina laboratory instruments with professional services direct from the manufacturer.
Get hands-on NGS and microarray training from expert instructors. We also offer live or self-paced online courses and other educational resources.
Access a full suite of accurate, expedient solutions ranging from instrument service plans to qualification services and training.
The MiSeqDx Instrument is intended for targeted sequencing of DNA libraries from human genomic DNA extracted from peripheral whole blood, formalin-fixed, paraffin-embedded (FFPE) tissue, or embryonic tissue, when used with in vitro diagnostic (IVD) assays performed on the instrument. The MiSeqDx Instrument is not intended for whole genome or de novo sequencing. The MiSeqDx Instrument is to be used with registered and listed, cleared, or approved IVD reagents and analytical software.
The MiSeqDx Instrument is intended for targeted sequencing of DNA libraries from human genomic DNA extracted from peripheral whole blood or formalin-fixed, paraffin-embedded (FFPE) tissue, when used with in vitro diagnostic (IVD) assays performed on the instrument. The MiSeqDx Instrument is not intended for whole genome or de novo sequencing. The MiSeqDx Instrument is to be used with registered and listed, cleared, or approved IVD reagents and analytical software.